Meet our Microcirculation Laboratory team members.
Team Members

Steven S. Segal, PhD
Principle investigator
Bio
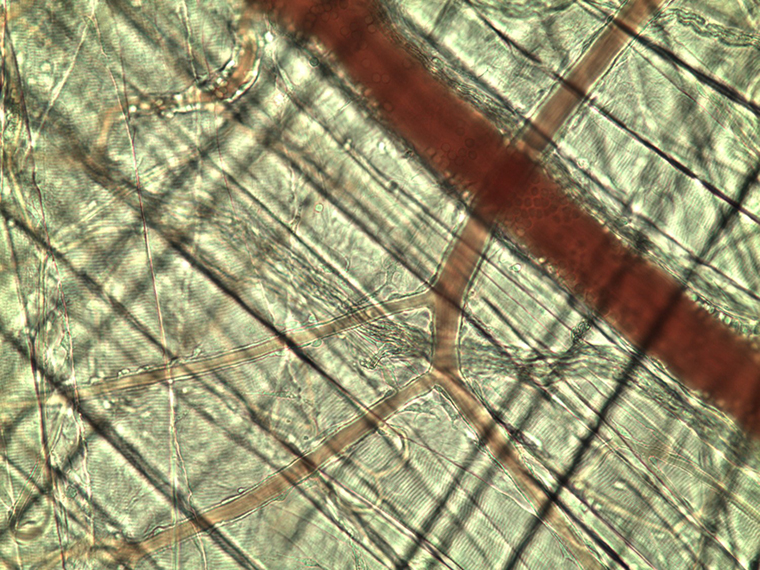
Research in Dr. Segal’s laboratory centers on mechanisms of blood flow regulation in the microcirculation. Longstanding interests have explored the coupling between tissue metabolic demand and functional vasodilation. Our studies of the underlying signals have resolved roles for electrical coupling and calcium waves among endothelial cells and smooth muscle cells in regulating local perfusion.
Current research centers on how acute injury affects the microvasculature and how vascular networks recover the ability to control local blood flow. In skeletal muscle, we are studying the nature of crosstalk between myofibers and microvessels during regeneration. Using genetic manipulation of myogenesis and angiogenesis in transgenic mice, we are gaining insight into how respective tissue components interact during regeneration to restore the structure and function of skeletal muscle - and what goes wrong when respective processes are disrupted.
Cell death upon reperfusion is an outcome of thrombotic stroke, myocardial infarction, and transplantation surgeries, attributable to the formation of reactive oxygen species (ROS) that disrupt cell membranes and integral proteins. In resistance arteries of skeletal muscle and brain exposed to the ubiquitous ROS, hydrogen peroxide, smooth muscle cells are more susceptible to injury than are endothelial cells, particularly in males. Remarkably, during chronic exposure to oxidative stress (as occurs during aging or eating a typical western-style diet), smooth muscle cells develop resilience to injury. Gaining new insight into cellular crosstalk during regeneration and of mechanisms underlying cellular resilience to oxidative stress will advance new translational therapies for regenerative medicine in restoring the quality of life in patients.

Charles Norton, PhD
Research Assistant Professor
Bio
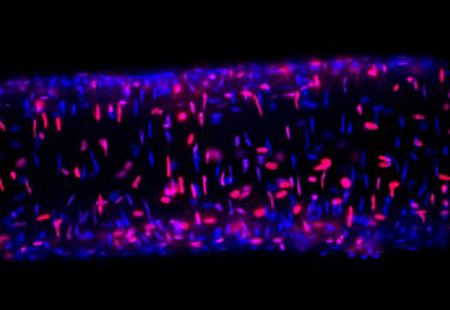
Charles is interested in understanding how conditions of acute oxidative stress, which occur during stroke or myocardial infarction, lead to endothelial and smooth muscle cell death in the arterial wall. Remarkably, conditions of chronic oxidative stress such as advanced age and western style diet increase the resilience of cells exposed to acute oxidative stress. Furthermore, arteries from females are less susceptible to acute oxidative stress than males. Charles seeks to understand the mechanisms that mediate this protection to provide insight into maintaining blood flow control following ischemic injury.
His secondary research interest centers on understanding mechanisms of pulmonary fibrosis-induced pulmonary hypertension. Pulmonary fibrosis is a respiratory disease with poor prognosis and limited therapy. Der to the strong correlation between the mortality in pulmonary fibrosis and the development of pulmonary hypertension, his research hopes to understand the mechanisms that lead to pulmonary hypertension during this disease.

Nicole Jacobsen, PhD
Post-doctoral Fellow
Bio
jacobsenn@health.missouri.edu
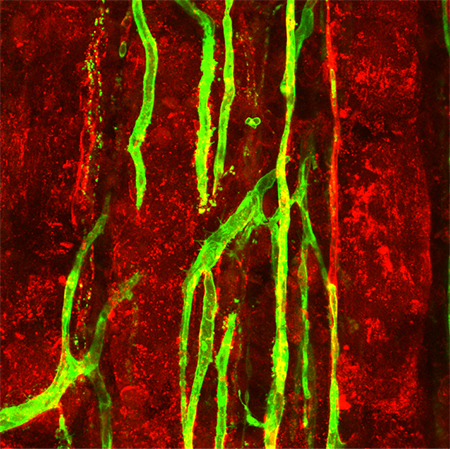
Acute injury to adult skeletal muscle triggers an inflammatory response, kills myofibers, and destroys capillary networks. While the resident muscle stem cells (satellite cells) are necessary for myofiber regeneration, the coordinated recovery of microvascular structure and function is essential for the restoration of intact functional muscle tissue. Despite the integral role of the microcirculation in supporting muscle function, data on the extent to which microvessels regenerate after tissue injury are scant.
Building on Nicole’s scientific background in cell-cell communication in vascular growth control, she studies network-wide perturbations in structure and function of the microcirculation during skeletal muscle regeneration. Using transgenic mouse models, Nicole identifies previously unrecognized interactions between cells of the microvessel wall and myogenic cells that are essential to effective tissue regeneration.
Education & Training
- Undergraduate: University of Arizona; BS in Nutritional Sciences
- Graduate: University of Arizona; PhD in Physiological Sciences “Proliferative and channel properties of gap junction proteins”
Awards & Honors
- NIH F32 Postdoctoral Fellowship 2021-2023
- AHA Predoctoral Fellowship 2016-2017
- Herbert E. Carter Travel Award, Graduate Interdisciplinary Programs 2016
- NIH T32 Interdisciplinary Training in Cardiovascular Research Predoctoral Fellowship 2013-2015
- Ruth R. Cowden Scholarship, College of Agriculture and Life Science 2009-2012
- Wildcat Award for Excellence Scholarship 2008-2012
- Arizona Board of Regents High Honors Scholarship 2008-2012

Aaron Morton, PhD
Post-doctoral Fellow
Bio
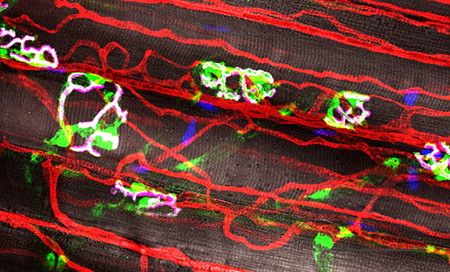
Skeletal muscle comprises roughly 40% of body mass and is important for locomotion and breathing. To support muscle fiber function, blood vessels indwell skeletal muscle to provide oxygen and nutrients as well as remove waste generated during metabolic activity, while motor nerves innervate muscle myofibers to induce contraction upon demand.
Anatomists have long known that both nerves and blood vessels share a similar branching architecture. In addition, developmental biologists have described strong interdependence of neural and vascular tissues during development. However, it is unknown if this neurovascular interdependence persists in the adult following skeletal muscle injury.
Utilizing both chemical and mechanical methods to induce muscle injury in a variety of murine, transgenic models, Aaron investigates neurovascular relationships during regeneration and the enhancement of this neurovascular regeneration through biomaterial applications.
Education & Training
- Undergraduate: Harding University in Searcy, AR
- Graduate: Master's from the University of West Florida in Pensacola, FL; PhD from the University of Florida in Gainesville, FL
Awards & Honors
- American Physiological Society IPE best poster award winner 2020
- Cardiovascular Day poster award winner 2020
- Neuromuscular Plasticity Summit poster award 2018
- NIH T32 Neuromuscular Plasticity Pre-doctoral fellowship 2016
- Jane Adams Edmonds Endowed PhD Fellowship University of Florida 2013
- UWF Exercise Science Graduate Student of the Year 2012

Rebecca Shaw
Research Specialist
Bio
Rebecca began her career at University of Missouri in the lab of Dr. Christine Heaps, under the direction of Dr. Douglas Bowles, in the School of Veterinary Medicine’s Biomedical Sciences Department in 2003. The focus of our work was smooth muscle cell proliferation and atherosclerosis in a porcine model.
Rebecca started work with Dr. Segal in 2010. Past projects focused on cell-cell coupling and expression of calcium channels in endothelial cell tubes. Much of her current work in our lab focuses on the study of endothelial and smooth muscle cell type differences in response to stress in the microvasculature of the brain. Using isolated whole vessel preps, she looks at responses to reactive oxygen species (ROS; exemplified by hydrogen peroxide) in the cell layers of mouse cerebral arteries.
Education & Training
- Undergraduate: University of Missouri-Columbia, BS Animal Sciences
Awards & Honors
- MU Staff Development Award 2019

Michael Nguyen
Undergraduate Research Assistant
Bio
Michael is an undergraduate student majoring in Biological Science with an emphasis in Medical Science and Human Biology with a pre-medicine career track. He discovered an interest in doing research during his time volunteering as an EMT, after realizing that besides the clinical aspect of medicine, he is interested in seeking a deeper understanding at the mechanistic level of human physiology. This led him to seek out and join Dr. Steven Segal’s laboratory.
He is currently mentored by Nicole Jacobsen, PhD, a post-doctoral fellow in the Segal laboratory, learning the foundational laboratory skills such as genotyping samples, imaging, and cross-section analysis. Michael applies those skills to investigate cross-talk between muscle fibers and blood vessels during myogenesis and angiogenesis post-injury.





