The overall research goal of our laboratory is to identify efficient therapeutic targets for the complex, life-threatening and sexually dimorphic aortic vascular disease: abdominal aortic aneurysms (AAA). AAA is an asymptomatic, permanent dilation of abdominal aorta which often causes death by aortic rupture in patients. AAA formation involves a complex process of destruction of the aortic media and supporting lamina through degradation of extracellular matrix proteins, elastin and collagen.
Among various risk factors, male sex has emerged as a positive risk factor. Incidence of AAAs in both humans and mice is 4–5 fold greater in males than females, and women acquire aggressive aneurysm growth and rupture at small AAA sizes when compared to men. Despite this oddity, the mechanisms driving sexual dimorphism of AAA have not been defined. The current available therapy is restricted to surgical repair, highlighting the need to explore mechanistic insights into AAA to develop effective, non-surgical therapeutics. The primary focus of our research is to study the functional contribution of cytoskeletal structural proteins, smooth muscle cell (SMC) homeostasis and extracellular matrix (ECM) protein stability to sexual dimorphism and aortic structural wall integrity during AAA development.
Role of calpains in abdominal aortic aneurysms
By using novel inducible calpain-2 deficient mice, we have examined the functional role of cytoskeletal structural protein stability on AAA formation.
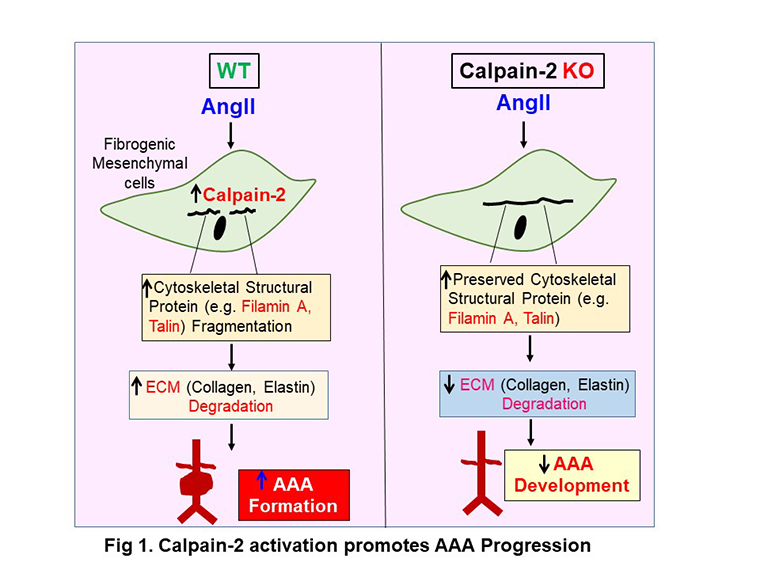
Calpains are intracellular proteases that uniquely target cytoskeletal structural proteins. To delineate the functional contribution of cytoskeletal structural proteins and calpain-2 in AAA pathology, we utilized a well-established mouse model of angiotensin II (AngII)-induced aortic aneurysms in male mice developed by Dr. Daugherty and Dr. Cassis, at the University of Kentucky. Using this animal model, we have demonstrated that pharmacological inhibition of calpain-2 completely attenuates AngII-induced AAA development in mice.
To further explore the molecular mechanisms by which calpain-2 activation promotes AAA formation, we have developed unique reagents, including transgenic, whole body or conditional cell-specific calpain-2 deficient mice. Using these novel reagents, we demonstrated that mesenchymal stem cell-derived calpain-2 promotes AAA formation in mice by promoting cytoskeletal structural fragmentation and aortic vascular medial destruction (Fig 1). Furthermore, inducible depletion of calpain-2 prevented rupture of established AAAs in male mice. Studies are in progress to understand the association of obesity accelerated perivascular adipose tissue inflammation on AAAs. We believe that calpain mediated microvesicles/exosomes secreted from the surrounding perivascular adipose tissue accelerates aortic vascular inflammation and promotes susceptibility to develop aortic aneurysms.
Role of miR-146a-Beclin-1 axis in abdominal aortic aneurysms
miR-146a is well known to regulate inflammatory and auto-immune processes under various disease conditions, including cardiovascular diseases by post-transcriptional repression of target genes. Autophagy is a self-regulatory process by which cells digest and recycle their cytoplasmic materials for energy purposes under stress. Beclin-1 is a gene essential for autophagy induction.
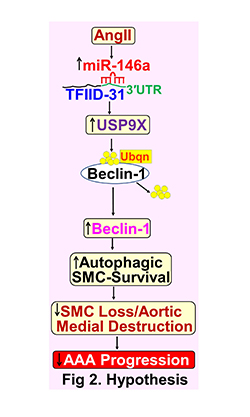
Using the novel animal model of Lysyl oxidase inhibition along with AngII infusion in normolipidemic mice, we identified that miR-146a is highly upregulated in AAA tissues. Using miR-146a deficient mice, in preliminary studies, we found that genetic deficiency of miR-146a accelerates AAA formation along with complete loss of autophagic Beclin-1 in male mice. In this project, our hypothesis is that activated miR-146a activation protects against AAA formation and progression by promoting Beclin-1-mediated aortic SMC homeostasis. Our working hypothesis is that miR-146a promotes USP9X-mediated Beclin-1 deubiquitination in aortic SMCs by repressing TFIID-31 via three prime untranslated region (3′UTR) binding, which in turn promotes autophagic-SMC survival (Fig 2).
These events ultimately suppress AngII-induced SMC-rich aortic medial destruction and AAA progression. We will address this hypothesis using recently developed unique reagents including conditional cell-specific miR-146a or Beclin-1 deficient mice, and lenti or adenoviral mediated miR-146a overexpressing or knockdown cell systems. These studies will provide preclinical evidence that targeting miR-146a activation represents a novel therapeutic strategy against AAA propagation and thereby provides new insight for development of diagnostic and therapeutic strategies for this clinically devastating disease.
Sexual dimorphism of abdominal aortic aneurysms
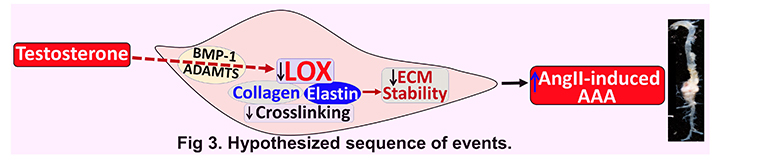
Both clinical and animal studies clearly highlight that males are at higher risk (>4-5 fold) than females in developing AAAs, and despite lower incidence, women acquire aggressive aneurysm growth and rupture at small AAA sizes compared to men. Using the novel animal model of Lysyl oxidase (LOX) inhibition along with AngII infusion in normolipidemic mice, for the first time we identified and demonstrated that inhibition of LOX abolishes sexual dimorphism and promotes AngII-induced AAAs in female mice as similar to male mice. In addition, we observed a sexual dimorphism in LOX activity in the abdominal aorta of mice. By performing gonadectomy in mice, our recent preliminary studies clearly demonstrated that testosterone plays a critical role in suppressing aortic LOX activity in male mice. Ongoing studies in our laboratory: 1) focus on how sex hormones regulate aortic LOX activity, and 2) test the novel hypothesis that testosterone-mediated suppression of aortic LOX activity is a critical contributor to sexual dimorphism of AAA (Fig 3), using the novel inducible LOX whole body or conditional (SMCs/fibroblasts) genetically deficient mouse models.
Role of Microtubules in abdominal aortic aneurysms
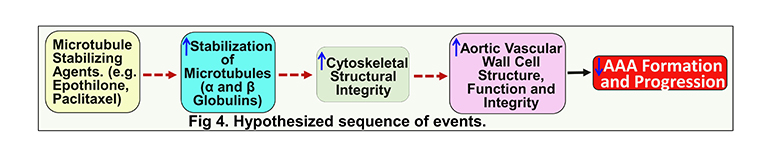
Microtubules are cytoplasmic tubules that serve as major structural components of the cellular cytoskeleton, and are involved in mitosis, intracellular transport and maintenance of cell structure and shape. Microtubules are composed of heterodimers of 2 globular proteins: alpha (α)- and beta (β)-tubulin subunits. Microtubules are stabilized by polymerization of α- and β-tubulin heterodimers. Microtubule stabilization likely represents a challenge for a cell to achieve cytoskeletal stability and healthy aging, especially for long-lived cells including aortic SMCs. Microtubule-stabilizing agents are emerging as an effective and viable approach to improve cell cytoskeletal stability under various disease conditions including neurodegenerative diseases and cancer. In our preliminary studies we identified that human and experimental AAAs are associated with decreased tubulin protein. However, virtually nothing is known about the functional contribution and/or stabilization of microtubules in AAA development, progression and sexual dimorphism. In this transformative project, we hypothesize that microtubule stabilization will preserve vascular cell cytoskeletal structural stability, thereby preventing AAA development and progression (Fig 4).
Research funding
1R01HL156957-01 Subramanian V (PI) 01/01/2022–12/31/2025
National Institute of Heart, Lung and Blood Institute (NIH/NHLBI)
University of Missouri start-up funds.





