Resolution of Inflammation
Our lab has pioneered the use of lipid mediators known as resolvins in salivary gland (SG) research, demonstrating that the resolvin D1 (RvD1) is biosynthesized in SGs. More specifically, we revealed the mechanisms by which RvD1 activates its receptor (ALX/FPR2) to block pro-inflammatory cytokines and promote tissue healing. Additionally, we demonstrated the degree to which the RvD1 receptor ALX/FPR2 is critical for resolution of inflammation in SGs, as mice lacking it display markedly increased reactivity to endotoxins. The significance of these findings is that we may have found a means to more effectively manage hyposalivation observed in Sjögren’s syndrome (SS) patients, with results to date demonstrating that systemic treatment with RvD1 of a mouse model for SS preserves and restores SG secretory function. To optimize this effect, we are currently working on a more targeted treatment involving local (retroductal) delivery of RvD1 directly to the SGs. Moreover, we believe that our translational studies, employing both the hALX/FPR2 knock-in mouse (i.e., a mouse carrying the human RvD1 receptor, hALX/FPR2) and human tissues, will provide insights that could well lead to clinically-relevant advancements in the near future.
Funding
5R01DE027884-01A1 Baker O.J (PI) 7/01/2018-4/30/2023
National Institute of Dental and Craniofacial Research (NIH/NIDCR)
A Targeted Approach to Managing Salivary Gland Inflammation Using Resolvins. The goal of this research program is to understand the mechanism(s) by which resolvins block secretory dysfunction in Sjögren’s syndrome.
Use of Fibrin Hydrogels to Promote Salivary Gland Regeneration

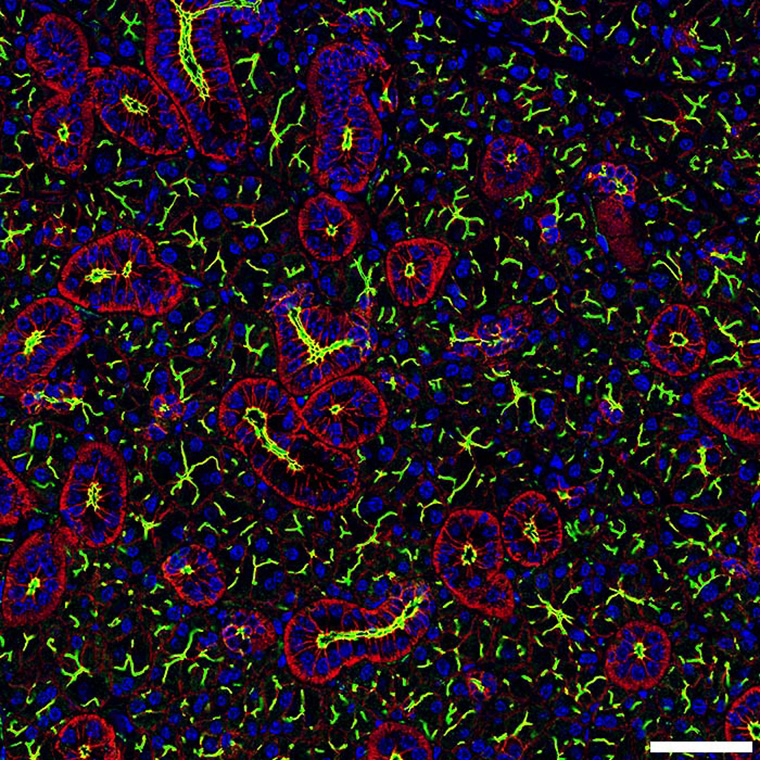
Shown is a confocal image is from a mouse submandibular gland. Read the Article.
We aim to design a matrix consisting of fibrin hydrogels (FH) linked to basement proteins and growth factors and introduce it into damaged SGs with the aim of forming new tissue and recovering lost secretory function. Specifically, we have demonstrated that the structural protein Laminin-1 (L1) promotes SG differentiation when polymerized within FH; however, because L1 is not optimal for translational research in light of its tumorigenic properties, we have designed alternate peptide sequences corresponding to four L1 regions that promote intact SG formation and conjugated them to FH. In so doing, we have retained the fertile characteristics of L1 without the associated dangers, thereby increasing the chances of producing new tissue with minimal risk for side effects. Furthermore, we have tested this L1-modified FH scaffold in damaged SGs and successfully applied it in vivo to restore SG function, thereby confirming that new and well-organized salivary tissue with intact secretion can be produced using our matrix. After more extensively demonstrating the effectiveness of our scaffold in mice, we will be in a position to extend these findings to human cells and then to human subjects. Eventually, we would like to combine gains made in scaffold building and tissue regeneration with our supplementary work in co-culturing nerve and salivary cells in an attempt to grow a functional SG.
Funding
1R01DE022971-01 Baker O.J (PI) 06/01/2021–05/31/2026
National Institute of Dental and Craniofacial Research (NIH/NIDCR)
The Use of Fibrin Hydrogels to Promote Salivary Gland Regeneration. The goal of this research is to promote salivary gland regeneration using fibrin hydrogels.
Cell Sheets
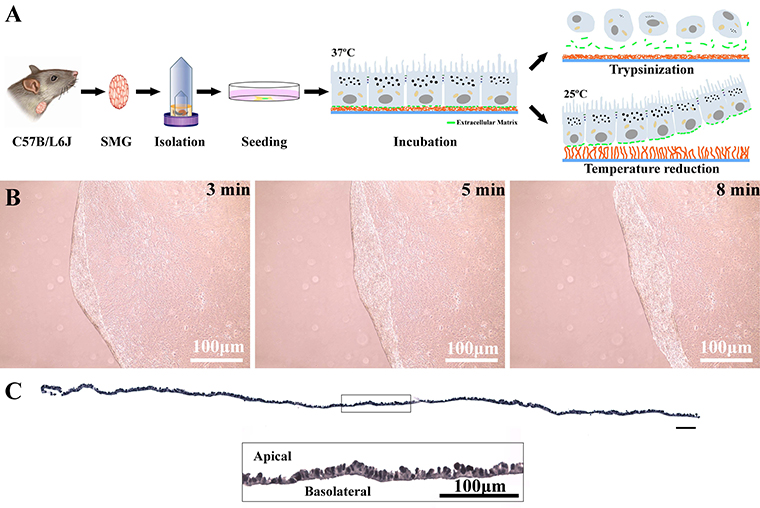
We are currently developing cell sheets from mouse and human salivary glands. With this technology, we believe that we will be able to grow more fully differentiated SG such that the functions of the acinar and ductal structures (i.e., saliva secretion and modification) will be observable together and in isolation, while also offering the possibility of both irrigation and innervation in the near future. Therefore, ongoing studies aim to characterize the cell sheet composition and cell fate once transplanted in vivo. Finally, we patented this cell sheet technology as applied to SG and believe it can ultimately be used for human tissue regeneration.
Funding
University of Missouri start-up funds.
Saliva Substitutes
Our lab is working to produce and test various saliva substitutes. The major breakthrough for development of saliva substitutes is creation of a novel process for purification of mucins at approximately one fifth the cost of the commercially available equivalent, making it possible to use this naturally occurring component as the basis of the saliva substitute to produce a product that we believe better mimics the feel and functions of the real thing than any of its predecessors.
Funding
TRIUMPH (Baker PI) 03/15/2021-2023
Building a Better Saliva Substitute. The goal of this proposal is to produce a novel saliva substitute and test it against the overwhelming leader in market for saliva substitutes overall.





