How is MBArC different from other grants?
MBArC funds translational research projects that have the potential to improve patient and health care. Awards of up to $200,000 for a year are used for product definition studies (e.g. feasibility studies, prototype development, proof-of-concept studies) comprised of critical go/no-go milestones. Applicants who are invited to submit a full proposal work with the MBArC Program Office and/or mentors to validate their unmet medical need, and establish a technology development and commercialization strategy. Funded projects are actively managed by the Program Office under a market-focused project management oversight and decision-making plan. Continued funding of an MBArC project is contingent upon successful and timely completion of project milestones.
Eligibility
Investigator: This funding opportunity is open to all faculty at MBArC participating institutions. Non-faculty applicants must identify a faculty member who is willing to sponsor their application. Having multiple investigators (one Principal Investigator and other Co-Investigators/Collaborators) is permitted but optional.
Product Type: All types of products in the human health space (e.g. therapeutic, diagnostic, device, tool, software) are eligible.
Intellectual Property Requirement: Applications should be based on ideas that originate from within a participating institution (making them potentially eligible for intellectual property protection) or university technologies with pending/issued patents or copyright. Technologies that are already licensed or optioned to a company are not eligible.
Proposal Evaluation
The research must have a direct application to an important unmet clinical need and the proposed solution, when commercialized, must have a benefit to patients and community health. Proposals are evaluated on the basis of scientific and clinical merit, potential health care impact and significance, experience of the investigators, timeline and pathway to commercialization as well as the likelihood of obtaining the additional funding required to fully translate the proposed solution.
MBArC Application Guidelines and Steps
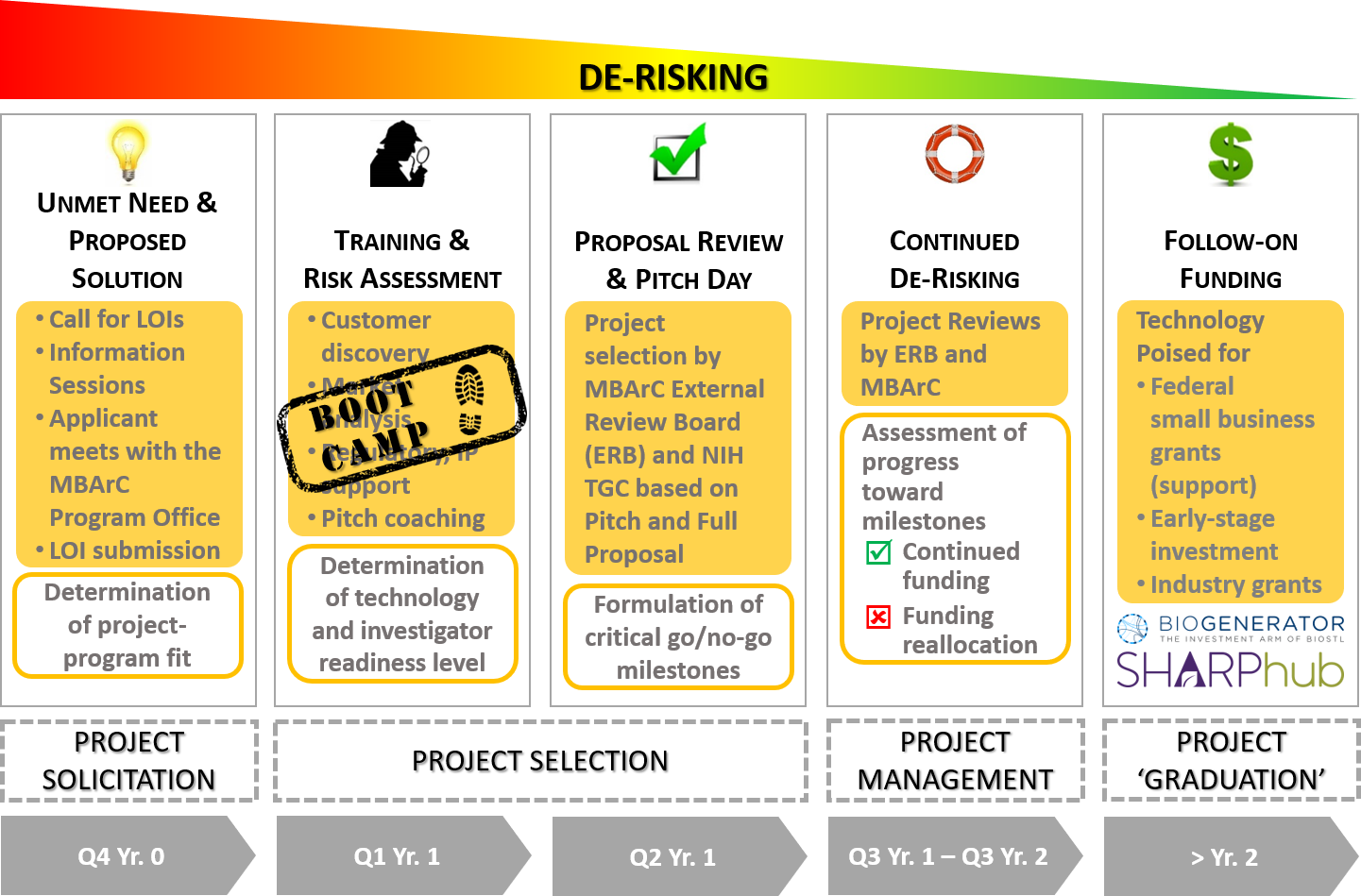
The multi-step application process includes submission of a Letter of Intent (LOI). LOIs are taken through a filtering process that includes a review by the MBArC Leadership Team. Applicants submitting LOIs that make it through the filtering process are invited to attend a commercialization Boot Camp that is now also offered in conjunction with MANGMT/ BIOL_EN 8200: Commercialization of Life Science Innovations, a graduate level course available to MU students. Applicants that complete Boot Camp and submit a viable full proposal are given the opportunity to make a Pitch to the MBArC External Review Board (ERB) for funding of the project. Projects recommended by the ERB are next reviewed by NIH REACH’s Technology Guidance Committee (TGC). The ERB makes the final decision on which projects are selected for funding. Funded projects are expected to start in Q3 of the funding year.
LOI Submission Guidelines
All investigators considering submission of a Letter of Intent (LOI) for an MBArC grant must complete this questionnaire and contact the Lead Program Manager Jaya Ghosh or call 573-882-0522 to schedule a meeting first.
Matching Funds Requirement for Non-MU Projects
MBArC supports each non-MU project with a maximum of $100,000 from the NIH award and the applicant is required to show at least an equal cash match (i.e., up to an additional $100,000) from non-federal sources (e.g., recipient institutions, foundations, for-profit investors, other state resources). When you contact the Program Office to set up your introductory meeting, include a Letter of Support from the institution or other non-federal source describing the latter’s willingness to commit 1:1 cash match of the direct costs of funding, if awarded. If you have additional questions about eligibility and matching funds requirement, contact the Program Office for assistance.
Commercialization Boot Camp
Commercialization Boot Camp is offered in conjunction with MANGMT/ BIOL_EN 8200: Commercialization of Life Science Innovations.
- Timeline: Q1 Yr. 1
- Objective: The primary objective of the Boot Camp is to help applicants answer two fundamental questions: 1) Does your envisioned product address a true unmet clinical need? AND 2) Is there a viable business opportunity? The second objective is to help applicants develop a robust proposal and pitch deck that best positions their idea for funding.
- Format: Invited speakers teach the basic principles of biomedical innovation and subject matter experts provide guidance on regulatory and reimbursement strategy, intellectual property and entrepreneurship. Applicants are also guided through four interactive home work sessions to identify stakeholders, define their value proposition and get-to-market strategy, develop their commercialization plan, and more. The Boot Camp and MANGMT/ BIOL_EN 8200 use the FROM LAB TO MARKET: The Process of Commercializing Medical Technologies and Biodesign Handbook, 1st Edition textbooks as guides.
- Boot Camp/Course Syllabus: Contact Jaya Ghosh for more details.
Full Proposal and Pitch Day
- Timeline: Q2 Yr. 1
- Objective: Convince the ERB that this grant will accelerate the transition of discovery originating from your academic research into a product or service that will improve human health by readying your technology for the next source of independent financing, such as the private sector, through a viable start-up company competitive for a small business award, or a licensing opportunity.
MBArC Funding
All investigators and collaborators on a funded project sign an Award Terms and Conditions Agreement prior to project start. Projects receive milestone-driven funding, and the MBArC Program Office meets with funded investigators on a monthly basis to track project progress. Investigators also meet with the ERB on a regular basis to report progress toward milestones. Based on these updates, the ERB makes go/no-go decisions on all funded projects. Non-progressing projects may be terminated.





